The hierarchy of evidence (1) is illustrated below:
Systematic Review: A review created after reviewing and combining all the
information from both published and unpublished studies (focusing on clinical
trials of similar treatments) and then summarizing the findings.
RCT: A study design that randomly assigns
participants into an experimental group or a control group. As the study is
conducted, the only expected difference between the control and experimental
groups is the outcome variable being studied.
Cross-sectional Studies: In a cross-sectional study, data are collected on the entire population
at a single point in time to examine the relationship between disease and
exposure. Therefore, they provide a snapshot of the frequency of a disease in a
population at a given point in time.
Case-control Studies: In a case-control study the study group is defined by the outcome disease, not by exposure to a risk factor. The study starts with the identification of a group of cases (individuals with a particular health outcome) in a given population and a group of controls (individuals without the health outcome) under investigation.
Case-control Studies: In a case-control study the study group is defined by the outcome disease, not by exposure to a risk factor. The study starts with the identification of a group of cases (individuals with a particular health outcome) in a given population and a group of controls (individuals without the health outcome) under investigation.
Non-randomised
studies: A study different from RCT in a
sense that the selection of subjects and controls is not randomised. Therefore,
despite the fact of being experimental studies, these feature lower on the
evidence hierarchy.
Cohort Studies: Cohort studies evaluate a possible
association between exposure and outcome by following a group of exposed
individuals over a period of time (often years) to see whether they develop the
disease or outcome of interest. A cohort is a group of individuals who share a
common characteristic, such as workers from the same factory.
Ecological Studies: Sometimes known as geographical or descriptive studies, can be used to
demonstrate patterns of disease and associated factors in a population.
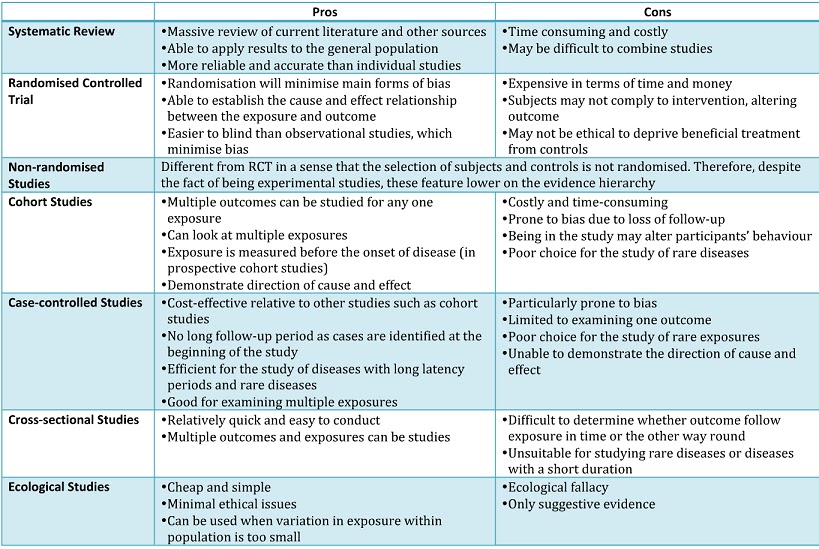
Below is a summary table (2) illustrating the respective pros and cons of each study type in the evidence hierarchy:
References:
1. NHMRC additional levels of evidence and grades for recommendations for developers of guidelines, National Health and Medical Research Council (Australia), 2009
2. Public Health Action Support Team (PHAST) [Internet] 2011. [cited 2013 June 5] . Available from: http://healthknowledge.org.uk/public-health-textbook/research-methods/1a-epidemiology

Excellent information on your blog, thank you for taking the time to share with us. You are helping others to grow their knowledge by sharing such a valuable information very helpful.
ReplyDeleteclinical evidence